Authors: Amer El-Samman, Claire Anderson
Discovering the Future of Flow Batteries with Machine Learning Techniques
Can AI Discover the Next Flow Battery?
One of the great advantages of AI technology in chemistry is its ability to speed up the prediction of important molecular behavior. Chemical behavior can be predicted with complicated quantum mechanics mathematics but it takes a tremendous amount of time to compute even for small molecules. This is bad for flow battery research. Many flow batteries are around medium-large sized molecules, thus even with the fastest supercomputers, we can achieve very little predictions efficiently. Meanwhile the combinatorial space of flow batteries is huge, we have billions, maybe even trillions, of possible flow batteries that may suit our purposes. AI can really aid in scanning this space for the most important molecules that suit the behavior we want. First let us explain what a flow battery is.
A flow battery is a type of electrochemical cell, typically rechargeable, where a dissolved redox pair are pumped into separated tanks. The battery is charged with electrical power which stores chemical potential across the two tanks tanks. This potential happens when one molecule, under the force of the introduced potential, loses electrons (anode) and becomes higher in energy, and another gains electrons (cathode). Overall, energy of the cell is higher and this chemical potential is stored until is ready for release.
When electrons are allowed to be released back, it spark the reverse reaction where electron travel back to original molecule that had them before discharging. The battery discharges energy in the process (which can be used to power up many large-scale processes) until the chemical potential that was stored is reduced down to zero. This means the cell has reached equilibrium, that is the battery is dead, and now requires recharging. Flow batteries are ideal for storing energy for large-scale infrastructure (i.e. not phone or laptop batteries).
There are several advantages of using flow batteries over conventional batteries with solid electroactive materials. Flow batteries independently scale the energy they can store with the size of the tanks used. They also scale the power (how fast it discharges and charges) depending on size of the stacks. They also tend to have long cycles and calendar life. However, flow batteries suffer from inferior cycle energy efficiency compared to lithium-ion batteries.
Machine Learning the Space of Optimal Bipyridine Derivatives for Flow Batteries
N,N-dimethyl bipyridine (above), here on referred to as bpy, is a promising molecule for flow battery for several reasons. Primarily, it is because it undergoes symmetric redox chemistry, i.e it acts as both the anode and the cathode in a flow battery
There are several reasons why this is advantageous. First, it is likely to be cheaper as it requires purchasing a single molecule. Second, there is no issue with cross-contamination between the two tanks as they would both contain the same material. Lastly, bpy releases and absorbs two electrons each time making the chemical potential for the flow battery likely to be high. For these reasons, it is interesting to investigate derivatives of this compound that will optimize its properties as a flow battery.
An optimal flow battery is one that 1) has the largest possible chemical potential window (within limits of the solvent’s potential window, as that can dissociate at the electrode), 2) is highly soluble in the solvent used in the flow battery, and 3) contains functional groups that are stable under external potential pressure (charging and discharging at the electrode) and that enable easy synthesis and characterization of the molecule.
We wrote a script that constructs various bpy derivatives with random nitrogen positioned at various ring positions, functional group substituents, and double bonds around the two rings. The technique follows several steps: 1) initialize a single hexagonal ring, 2) add random nitrogen on the one the ring, 3) add random number of functional group substituents at random positions, 4) add random number of double bonds at random positions, 5) make another symmetric ring constructed in steps 1-4, then 6) link the two rings with a linker out of a certain set, and finally 7) geometry optimize structure with force field MMFF94 model using RDKit python library. A few structures that come out of this algorithm are shown below:
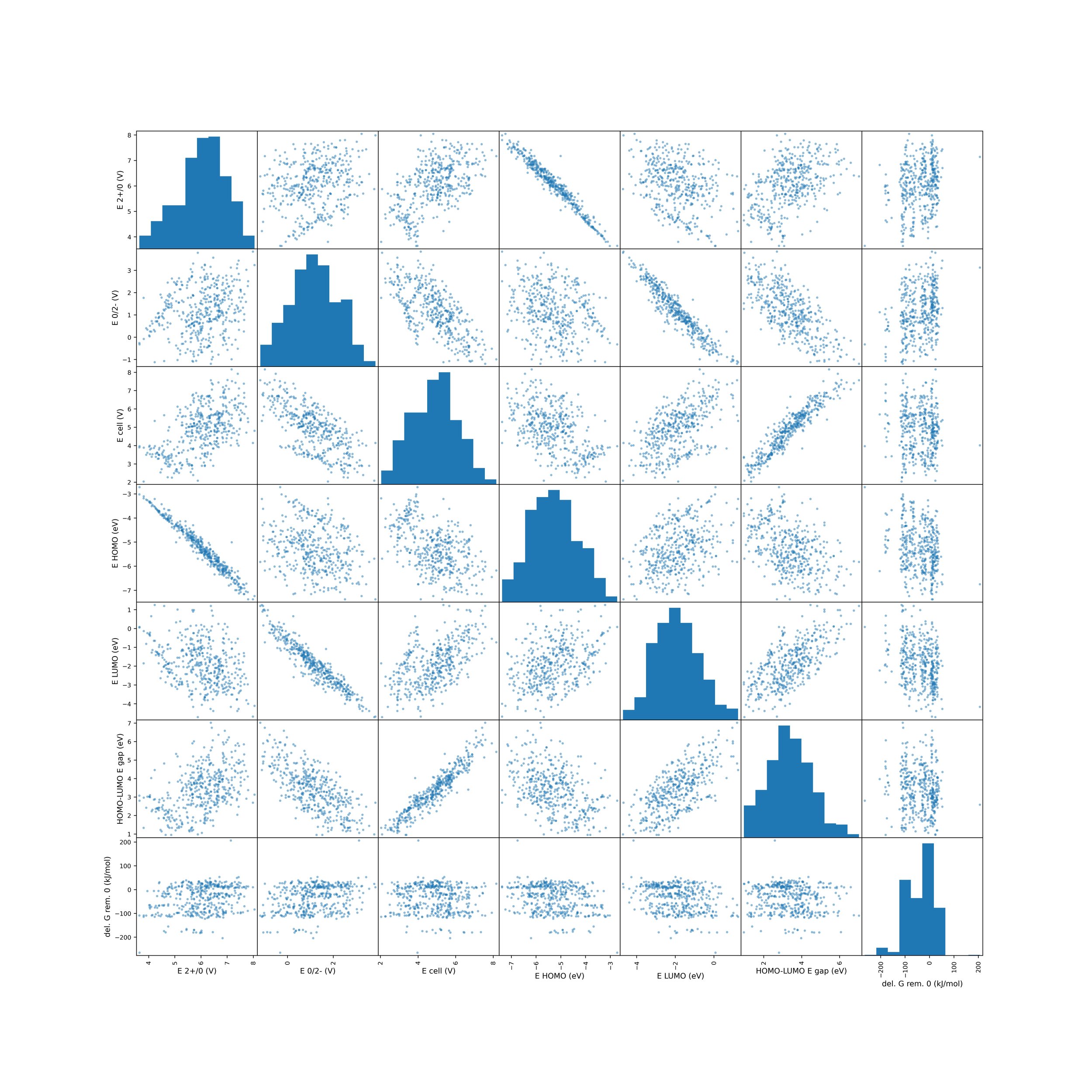
We left the combinatorial space open to a lot of possibilities, even many that seem entirely unstable and difficult to synthesize. The plan is to keep our options broad and diverse before using genetic algorithm that will combine the best structures in the data. To find the best structure from the diverse set, we will use a correlation matrix that compares 1) cell potential, 2) solvent-solubility and 3) functional group stability on the rings to find those molecules that are in the window of potential batteries. Using molecules in that window we will run a genetic algorithm machine learning technique which will combine the best possibilities to optimize structure for optimal flow battery properties. The correlation matrix is shown below.
The next step is to use the matrix above to identify an optimal set of molecules that will be put into a genetic algorithm. These are ones that satisfy having a good cell potential window, dissolve readily, and have stable functional groups. The genetic algorithm will then combine the best carbon framework and best functional group configurations. This will be done in future work and the results of this research will be updated.